Our Work on Dye Sensitized Solar Cells
(Dunbar P. Birnie, III
and Research Group)
Dye sensitized solar cells operate by absorbing light with dye molecules and having the dye molecules be part of an interesting electrical process that forces the electrons to run through an external circuit and deliver power to the outside circuit. The figure below is a cross-section that illustrates the fundamentals of these interesting cells.
Sunlight comes through a
transparent substrate (A), usually glass, that has a
transparent conductive oxide (B) on the surface as an electrode. The
light hits the dye molecules (D) that are soaked into and bonded to the surface
of a sponge of titanium dioxide 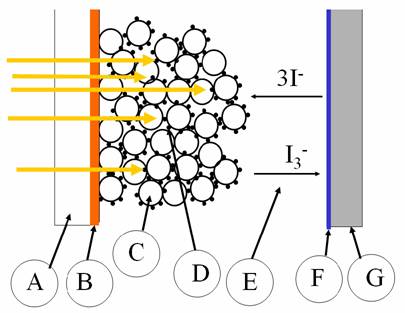 nanoparticles (C). The dye molecules absorb light which
puts an electron into an excited molecular orbital state and because the dye
is closely attached to the titanium oxide particle surfaces then the electron
is easily transferred into the solid and conducted by hopping from particle to
particle (moving leftward) to reach the transparent conductive oxide (B) which
is connected to the external world. The dye molecule is replenished by taking
electrons from an electrolyte system, which here is made by an iodide solution
(E). The two ion states allow for electrons to be conveyed through the solution
and connect with the other electrical
contact, a metal layer (F) on the back substrate (G).
nanoparticles (C). The dye molecules absorb light which
puts an electron into an excited molecular orbital state and because the dye
is closely attached to the titanium oxide particle surfaces then the electron
is easily transferred into the solid and conducted by hopping from particle to
particle (moving leftward) to reach the transparent conductive oxide (B) which
is connected to the external world. The dye molecule is replenished by taking
electrons from an electrolyte system, which here is made by an iodide solution
(E). The two ion states allow for electrons to be conveyed through the solution
and connect with the other electrical
contact, a metal layer (F) on the back substrate (G).
One VERY important aspect of the structure of these solar cells is that the two electrically conducting phases must inter-penetrate completely to allow for full closed circuit operation. That means that the titanium dioxide particles (C) must touch at enough contact points so that electrons can conduct from where the light absorption has happened to the left-side electrical contact. At the same time, the electrolyte with the iodide species must be able to infiltrate between the particles and bring electrons back to dye molecules throughout the entire nanoparticle sponge of titania. This requires a bi-continuous interconnected microstructure of grains and liquid between the grains.
Our recent research has been aimed at making microstructures that provide this kind of interconnectivity. We have been investigating templated materials that allow for the creation of interconnected porosity and leaves behind a skeleton of solid material that can provide locations for dye attachment. We have worked with reactive titanium precursors to form stable Ti-sols that can be mixed with stable polystyrene latex particles (left had view). When these solutions are coated onto a substrate and dried suitably then the polystyrene particles are forced into a close-packed array which is stuck together by the Ti precursors. Upon heat treatment then the Ti precursors react to form crystalline anatase in a structure shape that corresponds exactly to the crevices between the PS particles that were there. Simultaneously the polystyrene gets burned out, leaving behind the skeletal structure shown on the right.
 We
have adapted this process to accommodate the nanoparticle
sources that are more commonly used for making DSSCs.
For example, one frequently used source of titanium dioxide is the Degussa P25, which has a primary particle size in the range
of 20 nm. We have made templated structures where
these, already crystalline, titanium dioxide particles are then pushed into a
microstructure where the nano-pores between the
individual grains are still existent, thus providing many places for dye
attachment. At the same time the openings left behind where the PS particles
were still create an easy pathway for electrolyte infiltration and better
uniformity of conduction route during operation.
We
have adapted this process to accommodate the nanoparticle
sources that are more commonly used for making DSSCs.
For example, one frequently used source of titanium dioxide is the Degussa P25, which has a primary particle size in the range
of 20 nm. We have made templated structures where
these, already crystalline, titanium dioxide particles are then pushed into a
microstructure where the nano-pores between the
individual grains are still existent, thus providing many places for dye
attachment. At the same time the openings left behind where the PS particles
were still create an easy pathway for electrolyte infiltration and better
uniformity of conduction route during operation.
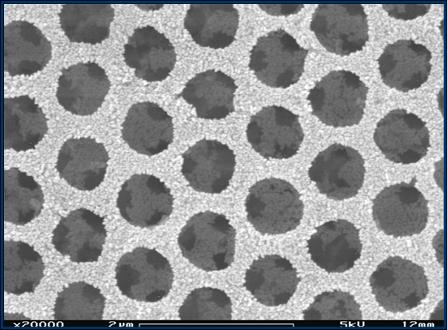 The
figure at the left shows a microstructure where we have made a templated P25 microstructure. The darker circles are holes
where 1-micron diameter polystyrene particles have been removed from the
structure. The much finer texture on the remaining solid parts comes from the nanoparticle titania.
The
figure at the left shows a microstructure where we have made a templated P25 microstructure. The darker circles are holes
where 1-micron diameter polystyrene particles have been removed from the
structure. The much finer texture on the remaining solid parts comes from the nanoparticle titania.
We have made these microstructures into solar cells and are continuing to investigate their utility. The initial studies, with an acetonitrile-based electrolyte, demonstrated that the dye molecules were more effective at converting sunlight when bound to this kind of templated structure, in comparison to a simple sintered P25 compact. We are continuing to evaluate how these microstructures can be used, especially in context with polymer and ionic liquid electrolytes that may have better applicability to printed electronics.
This work has been accepted for publication in Materials Letters. Our paper, authored by L. Qi and D. P. Birnie, III, is entitled Templated titania films with meso- and macroporosities and should appear in early 2007.
For further information about these microstructures, their application to solar cells, and possible application in batteries or other fields then please drop me a note by email.
© Copyright, 2006, Dunbar P. Birnie, III,